FDA Grants Profounda Inc. Orphan Drug Designation for treatment of Granulomatous Amebic Encephalitis
- Profounda
- Jun 28, 2017
- 2 min read

- GAE is a serious infection of the brain and spinal cord that typically occurs in persons with a compromised immune system.
- Few patients are known to have survived because of successful drug treatment. Early diagnosis and treatment might increase the chances for survival.
ORLANDO, Fla., June. 29, 2017 /PRNewswire/ -- Profounda, Inc. ("Profounda") announced today that it has received the US Food and Drug Administration's Orphan Drug Designation for the use of miltefosine to treat Granulomatous Amebic Encephalitis (GAE. Profounda licensed miltefosine (Impavido) from Knight Therapeutics (USA) Inc.
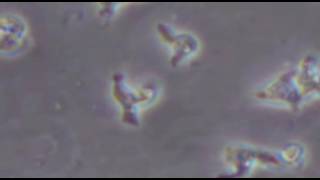
One of the causes of Granulomatous Amebic Encephalitis (GAE), a serious infection of the brain and spinal cord, is Balamuthia. The Balamuthia amoeba is thought to enter the body when soil or dust containing Balamuthia comes in contact with skin wounds and cuts, or is inhaled or gets in the mouth. The Balamuthia amoebas can then travel to the brain through the blood stream and cause GAE.
Amber Shoemake lost her son Leland Shoemake on September 25th, 2015 to this terrible disease and has set up a foundation in his name (lelandshoemakefoundation.com). GAE is often diagnosed only after death. However, it can be diagnosed by examining blood, cerebrospinal fluid, and tissue samples from a living patient as well. Amber is hoping to increase awareness of the deadly brain eating amoebas and has said “It took my son so I can talk about that evil all day”. In August of 2016, miltefosine played a role in helping a Florida teen survive a similar brain eating amoeba infection, Primary Amebic Meningoencephalitis (PAM) — Amebic Encephalitis.
Impavido® (miltefosine) is the first and only oral treatment approved by the FDA for visceral, mucosal and cutaneous leishmaniasis (a rare tropical parasitic disease). Leishmaniasis is the second leading parasitic cause of death in the world after malaria, and is rare in the USA, but we do see 15-30 cases per year. Impavido has also received orphan drug designation in treating Primary Amebic Meningoencephalitis (PAM), the brain infection caused by the water-born amoeba Naegleria fowleri and Acanthamoeba Keratitis, and is the first Rx product launched in the U.S. by Profounda, Inc.
Since minutes matter, Profounda has offered any hospital in the country a no charge consignment program in which there are now 15 participating hospitals across the country. Todd MacLaughlan, CEO of Profounda, says “While the number of patients dealing with these deadly amoebic infections is small, the devastating impact to families with loved ones in undeniable,” and further, “Increased awareness can lead to a faster diagnosis of GAE and PAM, and access to a potentially successful treatment is key”.
Recent Posts
See AllFDA Grants Profounda Orphan Drug Designation Approval for treatment of invasive candidiasis with Miltefosine - Invasive candidiasis is an...